Abstract
Plants convert external cues into mobile mRNAs to synchronize meristematic differentiation with environmental dynamics. These mRNAs are selectively transported to intercellular pores, plasmodesmata (PD), for cell-to-cell movement. However, how plants recognize and deliver mobile mRNAs to PD remains unknown. Here we show that mobile mRNAs hitchhike on organelle trafficking to transport towards PD. Perturbed cytoskeleton organization or organelle trafficking severely disrupts the subcellular distribution of mobile mRNAs. Arabidopsis rotamase cyclophilins (ROCs), which are organelle-localized RNA-binding proteins, specifically bind mobile mRNAs on the surface of organelles to direct intracellular transport. Arabidopsis roc mutants exhibit phenotype alterations and disruptions in the transport of mobile mRNAs. These findings suggest that ROCs play a crucial role in facilitating the systemic delivery of mobile mRNAs. Our results highlight that an RNA-binding protein-mediated hitchhiking system is specifically recruited to orient plant mobile mRNAs for intercellular transport.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data supporting the findings of this work are available within the article and supplementary information. Materials used in this study are available upon request.
References
Ham, B. K. & Lucas, W. J. Phloem-mobile RNAs as systemic signaling agents. Annu. Rev. Plant Biol. 68, 173–195 (2017).
Kehr, J., Morris, R. J. & Kragler, F. Long-distance transported RNAs: from identity to function. Annu. Rev. Plant Biol. 73, 457–474 (2022).
Kitagawa, M., Tran, T. M. & Jackson, D. Traveling with purpose: cell-to-cell transport of plant mRNAs. Trends Cell Biol. https://doi.org/10.1016/j.tcb.2023.05.010 (2023).
Banerjee, A. K. et al. Dynamics of a mobile RNA of potato involved in a long-distance signaling pathway. Plant Cell 18, 3443–3457 (2006).
Haywood, V., Yu, T. S., Huang, N. C. & Lucas, W. J. Phloem long-distance trafficking of GIBBERELLIC ACID-INSENSITIVE RNA regulates leaf development. Plant J. 42, 49–68 (2005).
Huang, N. C., Jane, W. N., Chen, J. & Yu, T. S. Arabidopsis thaliana CENTRORADIALIS homologue (ATC) acts systemically to inhibit floral initiation in Arabidopsis. Plant J. 72, 175–184 (2012).
Kim, M., Canio, W., Kessler, S. & Sinha, N. Developmental changes due to long-distance movement of a homeobox fusion transcript in tomato. Science 293, 287–289 (2001).
Lu, K. J., Huang, N. C., Liu, Y. S., Lu, C. A. & Yu, T. S. Long-distance movement of Arabidopsis FLOWERING LOCUS T RNA participates in systemic floral regulation. RNA Biol. 9, 653–662 (2012).
Huang, N. C., Luo, K. R. & Yu, T. S. Mobility of antiflorigen and PEBP mRNAs in tomato-tobacco heterografts. Plant Physiol. 178, 783–794 (2018).
Kim, G., LeBlanc, M. L., Wafula, E. K., dePamphilis, C. W. & Westwood, J. H. Genomic-scale exchange of mRNA between a parasitic plant and its hosts. Science 345, 808–811 (2014).
Notaguchi, M., Higashiyama, T. & Suzuki, T. Identification of mRNAs that move over long distances using an RNA-Seq analysis of Arabidopsis/Nicotiana benthamiana heterografts. Plant Cell Physiol. 56, 311–321 (2015).
Thieme, C. J. et al. Endogenous Arabidopsis messenger RNAs transported to distant tissues. Nat. Plants 1, 15025 (2015).
Xia, C. et al. Elucidation of the mechanisms of long-distance mRNA movement in a Nicotiana benthamiana/tomato heterograft system. Plant Physiol. 177, 745–758 (2018).
Turck, F., Fornara, F. & Coupland, G. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu. Rev. Plant Biol. 59, 573–594 (2008).
Li, C. et al. A cis element within flowering locus T mRNA determines its mobility and facilitates trafficking of heterologous viral RNA. J. Virol. 83, 3540–3548 (2009).
Ellison, E. E. et al. Multiplexed heritable gene editing using RNA viruses and mobile single guide RNAs. Nat. Plants 6, 620–624 (2020).
Yang, L. et al. Heritable transgene-free genome editing in plants by grafting of wild-type shoots to transgenic donor rootstocks. Nat. Biotechnol. 41, 958–967 (2023).
Luo, K. R., Huang, N. C. & Yu, T. S. Selective targeting of mobile mRNAs to plasmodesmata for cell-to-cell movement. Plant Physiol. 177, 604–614 (2018).
Romano, P. G., Horton, P. & Gray, J. E. The Arabidopsis cyclophilin gene family. Plant Physiol. 134, 1268–1282 (2004).
Kovalev, N. & Nagy, P. D. Cyclophilin A binds to the viral RNA and replication proteins, resulting in inhibition of tombusviral replicase assembly. J. Virol. 87, 13330–13342 (2013).
Hanhart, P. et al. Bioinformatic and expression analysis of the Brassica napus L. cyclophilins. Sci. Rep. 7, 1514 (2017).
Buxbaum, A. R., Haimovich, G. & Singer, R. H. In the right place at the right time: visualizing and understanding mRNA localization. Nat. Rev. Mol. Cell Biol. 16, 95–109 (2015).
Ivanov, S. & Harrison, M. J. A set of fluorescent protein-based markers expressed from constitutive and arbuscular mycorrhiza-inducible promoters to label organelles, membranes and cytoskeletal elements in Medicago truncatula. Plant J. 80, 1151–1163 (2014).
Yang, L. et al. Myosins XI modulate host cellular responses and penetration resistance to fungal pathogens. Proc. Natl Acad. Sci. USA 111, 13996–14001 (2014).
Heinlein, M. Plant virus replication and movement. Virology 479-480, 657–671 (2015).
Cai, G., Faleri, C., Del Casino, C., Emons, A. M. & Cresti, M. Distribution of callose synthase, cellulose synthase, and sucrose synthase in tobacco pollen tube is controlled in dissimilar ways by actin filaments and microtubules. Plant Physiol. 155, 1169–1190 (2011).
Wang, F. et al. Phosphorylation and ubiquitination of dynamin-related proteins (AtDRP3A/3B) synergically regulate mitochondrial proliferation during mitosis. Plant J. 72, 43–56 (2012).
Wu, S. & Gallagher, K. L. The movement of the non-cell-autonomous transcription factor, SHORT-ROOT relies on the endomembrane system. Plant J. 80, 396–409 (2014).
Scheuring, D. et al. Multivesicular bodies mature from the trans-Golgi network/early endosome in Arabidopsis. Plant Cell 23, 3463–3481 (2011).
Lee, J. Y. et al. A plasmodesmata-localized protein mediates crosstalk between cell-to-cell communication and innate immunity in Arabidopsis. Plant Cell 23, 3353–3373 (2011).
Marondedze, C., Thomas, L., Gehring, C. & Lilley, K. S. Changes in the Arabidopsis RNA-binding proteome reveal novel stress response mechanisms. BMC Plant Biol. 19, 139 (2019).
Reichel, M. et al. In planta determination of the mRNA-binding proteome of Arabidopsis etiolated seedlings. Plant Cell 28, 2435–2452 (2016).
Heard, W., Sklenar, J., Tome, D. F., Robatzek, S. & Jones, A. M. Identification of regulatory and cargo proteins of endosomal and secretory pathways in Arabidopsis thaliana by proteomic dissection. Mol. Cell. Proteom. 14, 1796–1813 (2015).
Chou, I. T. & Gasser, C. S. Characterization of the cyclophilin gene family of Arabidopsis thaliana and phylogenetic analysis of known cyclophilin proteins. Plant Mol. Biol. 35, 873–892 (1997).
Tian, L. et al. Zipcode RNA-binding proteins and membrane trafficking proteins cooperate to transport glutelin mRNAs in rice endosperm. Plant Cell 32, 2566–2581 (2020).
Ashley, J. et al. Retrovirus-like Gag protein Arc1 binds RNA and traffics across synaptic boutons. Cell 172, 262–274 e211 (2018).
Cai, Q. et al. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 360, 1126–1129 (2018).
Vaten, A. et al. Callose biosynthesis regulates symplastic trafficking during root development. Dev. Cell 21, 1144–1155 (2011).
Dimou, E. & Nickel, W. Unconventional mechanisms of eukaryotic protein secretion. Curr. Biol. 28, R406–R410 (2018).
Petit, J. D., Li, Z. P., Nicolas, W. J., Grison, M. S. & Bayer, E. M. Dare to change, the dynamics behind plasmodesmata-mediated cell-to-cell communication. Curr. Opin. Plant Biol. 53, 80–89 (2019).
Liao, Y. C. et al. RNA granules hitchhike on lysosomes for long-distance transport, using Annexin A11 as a molecular tether. Cell 179, 147–164 (2019).
Pohlmann, T., Baumann, S., Haag, C., Albrecht, M. & Feldbrugge, M. A FYVE zinc finger domain protein specifically links mRNA transport to endosome trafficking. eLife 4, e06041 (2015).
Yang, L. et al. m5C methylation guides systemic transport of messenger RNA over graft junctions in plants. Curr. Biol. 29, 2465–2476 (2019).
Zhang, W. et al. tRNA-related sequences trigger systemic mRNA transport in plants. Plant Cell 28, 1237–1249 (2016).
Ham, B. K. et al. A polypyrimidine tract binding protein, pumpkin RBP50, forms the basis of a phloem-mobile ribonucleoprotein complex. Plant Cell 21, 197–215 (2009).
CRISPR-P 2.0: an improved CRISPR/Cas9 tool for genome editing in plants. Huazhong Agricultural University http://crispr.hzau.edu.cn/CRISPR2/ (2016).
Acknowledgements
We thank H. Li, S.-H. Wu and C.-M. Ho for critical comments on the manuscript, and Y. Wu for helping in ROC characterization. M.-J. Fan and IPMB cell biology core facility for providing technique support on confocal microscopy. This work was supported by grants from National Science and Technology Council, Taiwan (grant no. MOST111-2311-B-001-024-MY3, to T.-S.Y.)
Author information
Authors and Affiliations
Contributions
K.-R.L., N.-C.H. and T.-S.Y. designed the experiments. K.-R.L. performed live-imaging experiments. N.-C.H. performed the RIP assay, Arabidopsis seedling grafting and RT–qPCR analyses. N.-C.H. and Y.-H.C. characterized roc mutants. Y.-W.J. performed the western blot analysis. K.-R.L. and T.-S.Y. wrote the manuscript with inputs from N.-C.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Manfred Heinlein, Munenori Kitagawa and James Westwood for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The fluorescence-based mRNA live-imaging system.
(a) Illustration of the mRNA imaging system. The free form of nucleus-localized MS2FD-GFP is restricted in the nucleus but translocates to the cytosol upon interacting with mRNA containing the MS2-recognition stem loop (FTSL24). (b–e) Images of the cells co-expressing MS2FD-GFP and FT mRNA (b), FTSL24 mRNA (c), RFP mRNA (d), or RFPSL24 mRNA (e). MS2FD-GFP-labeled mobile FTSL24 mRNA displayed a punctate distribution at the cell periphery (c) but non-mobile RFPSL24 mRNA displayed a homogenous cytoplasmic localization (e). Scale bar =20 µm (b, d), or =10 µm (c, e).
Extended Data Fig. 2 Distribution of cytoskeleton network and organelle markers with chemical treatment.
(a) Distribution of Lifeact-mCherry-labeled actin microfilament network in cells treated with DMSO- or latrunculin B (LatB). Z-thickness = 31 µm (DMSO) or 29 µm (LatB). Scale bar = 20 µm. (b) Distribution of mCherry-MAP4-MBD labeled microtubule network in cells treated with DMSO- or oryzalin (Ory). Z-thickness = 21 µm (DMSO) or 29 µm (Ory). Scale bar = 20 µm. (c) Confocal images of cells co-expressing MS2FD-GFP-labeled FTSL24 mRNA and MAN49-mCherry-labeled Golgi. MS2FD-GFP-labeled FTSL24 mRNA (arrow) does not co-localize with MAN49-mCherry in cytosol. Nucleus (N) and cell periphery (arrowhead). Scale bar = 10 µm. (d, e) DMSO or Brefeldin A (BFA) treatment of cells co-expressing MAN49-mCherry, MS2FD-GFP and FTSL24. Note that BFA relocates MAN49-mCherry to ER (d), but had no effect on punctate distribution of MS2FD-GFP-labeled FTSL24 mRNA (e). Scale bar= 5 µm (d), or 10 µm (e).
Extended Data Fig. 3 Disruption of RAB5-positive compartments interfere mobile mRNA transport.
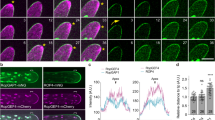
(a) Concanamycin A (CMA) treatment cells co-expressing MS2FD-GFP, FT mRNA (without stem-loops) and mCherry-RAB5. CMA treatment did not induce non-specific interaction between MS2FD-GFP and aggregated mCherry-RAB5. Z-thickness = 30 µm. Scale bar = 20 µm. (b) Statistic analysis of Fig. 2 by overlap coefficient analysis. Co-localization of MS2FD-GFP-labeled FTSL24 mRNA and mCherry-RAB5 under DMSO, CMA, or wortmannin (Wort) treatment were analyzed. N = 6 (DMSO), 4 (CMA), and 10 (Wort). Data are mean ± SD. p values derived from two-tailed Mann-Whitney U test are indicated. (c) CMA has no effects on mCherry-RAB5 protein accumulation. Western blot analysis of mCherry-RAB5 protein levels in cells treated with DMSO or CMA. Cytosolic glyceraldehyde-3-phosphate dehydrogenase (GAPC) was used as a loading control, correlated to Fig. 2a, b. (d) Detection of mCherry-RAB5 protein accumulation in RNA immunoprecipitation (RIP) analysis of mRNA associated with mCherry-RAB5-containing compartments, correlated to Fig. 2d. Cells co-expressing mCherry-RAB5 and FT or RFP mRNA showed comparable mCherry-RAB5 protein levels before (Input) and after (IP) immunoprecipitation with mCherry antibodies. (e) AGL24SL24 mRNA binds to RAB5-positive compartments, as indicated by colocalization of MS2FD-GFP-labeled AGL24SL24 mRNA with aggregated mCherry-RAB5-positive compartments (indicated by white arrows) after CMA treatment. Scale bar = 5 µm.
Extended Data Fig. 4 Colocalization of FTSL24 mRNA with ARA6-, ARA7- or RAB7-positive compartments.
(a–c) Confocal microscopy analysis of cells co-expressing MS2FD-GFP, FTSL24 mRNA with mCherry-ARA6 (a), mCherry-ARA7 (b), or mCherry-RAB7 (c). Scale bar = 10 µm. (d) RIP analysis of cells co-expressing FT with mCherry-RAB5 or mCherry-RAB7. Samples before (Input) or after (RIP) immunoprecipitation with mCherry antibodies were used for RT-PCR with FT specific primers to detect FT mRNA.
Extended Data Fig. 5 Cell-to-cell movement of putative endosome-localized RNA-binding proteins in Arabidopsis.
Transient co-expression of DsRed with (a) GFP-AT1G18080 or (b) GFP-AT5G47210 by particle bombardment of Arabidopsis leaf epidermal cells. Z-thickness = 13 µm (a) or 19 µm (b). Scale bar = 20 µm.
Extended Data Fig. 6 Expression pattern of Arabidopsis cytoplasmic ROCs.
(a) GUS expression driven by different ROC promoters in Arabidopsis seedlings. Scale bar = 1 mm. (b) GUS expression driven by ROC promoters in Arabidopsis root. Scale bar = 0.1 mm. (c) In planta level of ROC1, 2, 3, 5, and 6 mRNAs relative to UBIQUTIN CONJUGATION ENZYME mRNA level, set to 1. N = 3 for all sets, data are mean ± SD.
Extended Data Fig. 7 Cytoplasmic ROCs (ROC1, 2, 3, 5, and 6) but not ER-localized ROC7 are mobile proteins.
Confocal microscopy analysis of GFP fusion proteins distribution in roots of Arabidopsis transformants harboring SUC2pro:GFP, SUC2pro:GFPER, or different SUC2pro:ROCs-GFP. The roots were counterstained with propidium iodide. Scale bar = 50 µm.
Extended Data Fig. 8 Detection of ROC3-GFP protein level on RNA immunoprecipitation (RIP).
Western blot analysis of ROC3-GFP protein level in RIP of cells co-expressing ROC3-GFP and FT or RFP mRNA, correlated to Fig. 4d. Comparable ROC3-GFP protein level before (Input) and after (IP) immunoprecipitation was detected with antibodies against GFP.
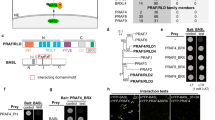
Extended Data Fig. 9 Characterization of Arabidopsis roc mutants.
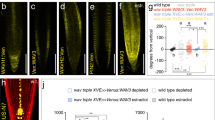
(a) DNA sequencing analysis of roc1 heterozygotic mutant generated by the CRISPR-Cas9 system. A 2-bp deletion, which results in a frameshift-sequence (indicated by an arrow), was detected in the roc1 mutant. The number labeled on WT sequences represents the nucleotide position after start codon. (b) Insertion site of T-DNA in roc mutant alleles. Numbers correspond to nucleotide position of T-DNA insertion relative to ATG (set to 1). Black blocks, exons; open blocks, untranslated regions. (c) RT-PCR analysis of ROC gene expression in corresponding T-DNA insertion lines. In the roc2 mutant, the amplified DNA fragment of ROC2 gene is indicated by an arrow. Actin was a loading control. (d) Short-day (SD) grown of Arabidopsis wild-type (Col) and roc1,2,3,5 quadruple mutants. The plants were grown in a growth camber with 8-h/16-h day/night cycle for 119 days. (e) Flowering time (indicated as total rosette leaf number) of Col and roc1,2,3,5 under SD conditions. N = 16 (Col) and 17 (roc1,2,3,5), data are mean ± SD. p value derived from two-tailed unpaired t test is indicated.
Extended Data Fig. 10 Control experiments correlated for Fig. 6.
(a) Nuclear MS2FD-GFP fluorescence intensity in cells subjected for particle bombardment experiment, correlated to Fig. 6f. N = 42 (Col), 27 (roc1,2,5), 39 (roc1,3,5), and 28 (roc1,2,3,5), data are mean ± SD. p = 0.55, one-way ANOVA (Kruskal-Wallis test). (b) Relative expression of FTSL24 mRNA in transgenic lines serve as stocks in grafting experiments, correlated to Fig. 6g, h. N = 3 for all sets, data are mean ± SD.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2 and source data 1–4.
Supplementary Video 1
Co-localization of MS2FD–GFP-labelled FTSL24 mRNA and mCherry-labelled actin microfilament (Lifeact) during transport. N, nucleus. Time is in minutes:seconds.
Supplementary Video 2
Transport-arrested MS2FD–GFP-labelled FTSL24 mRNA and MAN49–mCherry-labelled Golgi in cells with LatB treatment. Time is in minutes:seconds.
Supplementary Video 3
Transport of FTSL24 mRNA along mCherry–MAP4–MBD-labelled microtubule. Cell boundaries are indicated by dashed lines. Time is in seconds.
Supplementary Video 4
Co-localization of MS2FD–GFP-labelled FTSL24 mRNA and enlarged mCherry-labelled RAB5 compartments in cells with CMA treatment. Time is in minutes:seconds.
Supplementary Video 5
Dissociation of MS2FD–GFP-labelled FTSL24 mRNA and enlarged mCherry-labelled RAB5 compartments in cells with Wort treatment. Time is in minutes:seconds.
Supplementary Video 6
Three-dimensional reconstruction of the cellular region shown in Fig. 4c–e. Grid size, 10 µm.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, KR., Huang, NC., Chang, YH. et al. Arabidopsis cyclophilins direct intracellular transport of mobile mRNA via organelle hitchhiking. Nat. Plants 10, 161–171 (2024). https://doi.org/10.1038/s41477-023-01597-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-023-01597-5